The 26th Annual Fall EIS Short Course:
Electrochemical Impedance Spectroscopy:
Theory – Applications – Laboratory Instruction
Dates: November 10-14, 2014
Where: Houston, Tx
 Course Overview
Course Overview
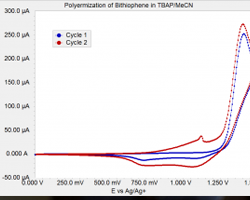
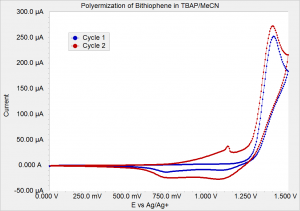
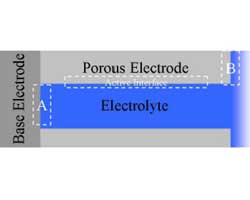
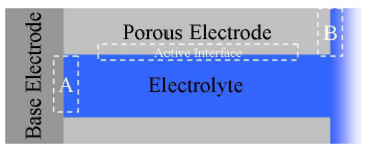
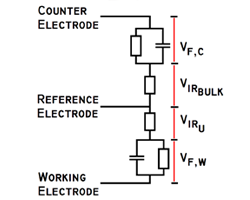
Impedance spectroscopy is an extremely powerful non-destructive investigative technique that can obtain essential information about interfacial and bulk material parameters through the use of low energy, time varying electrical excitation. When applied to an electrochemical system, electrochemical impedance spectroscopy (EIS) can provide information on reaction parameters, corrosion rates, oxide characteristics and integrity, surface porosity, coating integrity, mass transport, and many other electrode/interface characteristics. EIS is possibly one of the most powerful methods available in electrochemistry today and is utilized in research and development in essentially every technical sector, e.g., transportation and infrastructure, batteries and fuel cells, medicine, among many others. However, effective utilization of this method has been hindered by the lack of a comprehensive and cohesive explanation of the theory, measurement, and analysis techniques. This course on EIS has been developed to fill this void.
This short course, now in its 26th year, was first taught in 1988 in Charlottesville, Virginia. It has recently had the tremendous fortune of moving to the vibrant and technologically rich city of Houston, Texas. Please read more about our course on this website, or contact us either by e-mail or telephone.
Course Objective […]











 Course Overview
Course Overview