Introduction
This Application Note presumes that you have a basic understanding of potentiostat operation. If you are not that knowledgeable concerning electrochemical instrumentation, please read Potentiostat Fundamentals before continuing. Experienced potentiostat users may skip the primer and read on.
It’s only natural that electrochemists concentrate on the working electrode. After all, reactions at the working electrode are  what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
For reliable reference electrode performance, you should assign a “Lab Master” and treat it very, very carefully so it can serve as a standard for your other reference electrodes. Never use the Lab Master in an actual experiment. The only purpose of the Lab Master is to serve as a check for the other reference electrodes. If a reference electrode is suspected to be bad, you can check the potential versus the Lab Master. You can do that with a voltmeter, or with your Gamry Potentiostat by running and open circuit potential. If the potential difference is less than 2-3 mV, it’s OK. If it’s higher than 5 mV, it needs to be refreshed or discarded.
[…]
 What’s important in the selection of a potentiostat? That depends greatly on its intended use. This page addresses an often misunderstood term.
What’s important in the selection of a potentiostat? That depends greatly on its intended use. This page addresses an often misunderstood term.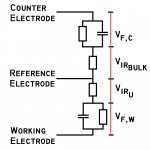

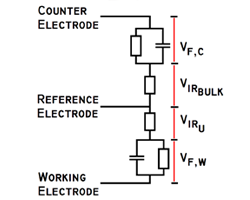


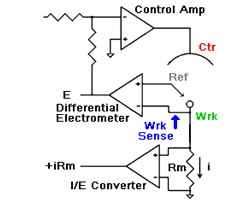
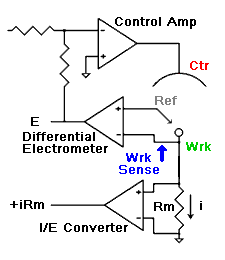 amperes. This scheme has been used by Gamry, PAR, Solartron, and perhaps others.
amperes. This scheme has been used by Gamry, PAR, Solartron, and perhaps others.
 what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.