Often we find a potential listed in the literature quoted against a different reference electrode than the one we favor, or we would like to convert the potential to a more commonly used reference electrode for publication.
 A student emailed me:
A student emailed me:
“My experiments involve measuring the redox potential relative to a saturated Ag/AgCl reference electrode. In order to obtain the redox potential relative to an SCE electrode, do I subtract 0.045 V from all the potentials which I obtained (using the Ag/AgCl reference electrode)?”
Here is my answer:
I always like to think of a potential line, with the potentials vs. NHE on the axis. I’ve drawn the axis below, with positive to the right (my Polarographer friends will eventually forgive me)
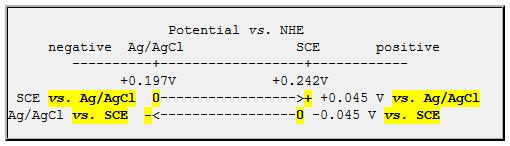
When you say “xy.zzy volts vs. a reference electrode”, you are defining where zero is on this line: “vs. SCE” defines the potential of the SCE as the reference zero.
If you use an Ag/AgCl reference electrode to measure the potential of electrode X, (which is ALSO an Ag/AgCl electrode!), you would report zero voltage difference (if both electrodes were functioning properly). From the little graphic, above, you should report the voltage of electrode X as 0.0V vs. Ag/AgCl or -0.045V vs. SCE.
So, the short answer is “You should SUBTRACT 45 mV from readings obtained with an Ag/AgCl,sat’d NaCl reference electrode in order to convert to potential vs. SCE.
You may wish to think of it as adding -45 mV, since this is potential of the reference electrode you actually used (Ag/AgCl) vs. the electrode you’d like to quote (SCE).
