Care of Porous Glass Frits
Porous Glass is often used at the end of a reference electrode or a bridge tube to allow electrical, ionic conductivity between the bulk of the solution and the internal filling solution, while preventing large scale convective mixing of the solutions. Porous Glass, or “thirsty glass” is a porous glass with a fairly low leak rate.
The Porous Glass frits, however, are not immortal!
To preserve their useful lifetime, they should be kept wet. If they are allowed to dry out, solid crystals can clog the narrow pores and increase the electrical resistance. In extreme cases, the Porous Glass can crack upon drying out.
When not in use, the reference electrode or bridge tube can be stored with the Porous Glass frit immersed in distilled water. Diffusion through the Porous Glass is fairly slow, and the internal filling solution will not be diluted, even upon a few weeks of storage.
An alternative is to replace the small plastic cap that was in place when the reference electrode was shipped.
Replacing a Porous Glass Frit
[…]

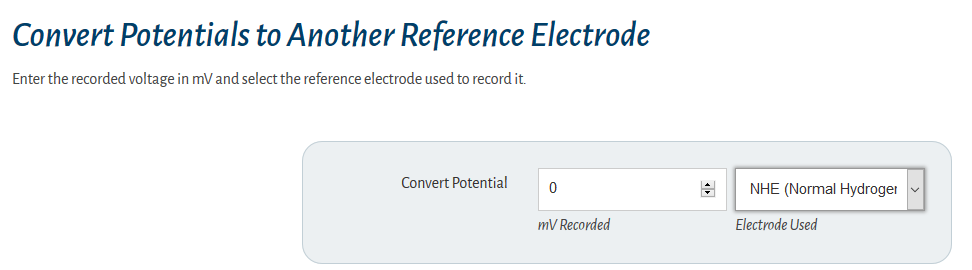




 what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
