EQCM Investigations
This Application Note is intended to provide the reader with a general framework for characterization of an electroactive polymer film. Electropolymerization is a convenient way to control film growth either through repeated cycling, potential steps, or current steps. Examination of the film redox behavior in monomer-free, fresh electrolyte provides insight on doping and dedoping of these polymer films.
Polybithiophene films were assembled by cycling an Au-coated 10 MHz quartz crystal between 0 and 1.5 V in the presence of 1 mM bithiophene solution containing 100 mM tetrabutylammonium perchlorate (TBAP) in acetonitrile (MeCN). Potentials are reported against a Ag/Ag+ pseudo-reference electrode. The Teflon® cell was outfitted with a Teflon o-ring to prevent swelling from the acetonitrile and placed inside a VistaShield™. The cell was connected to an eQCM 10M™ which was coupled to a Reference 600™. Both instruments were connected to a computer running Resonator™ version 5.67. Bithiophene, electropolymerizes via a two-electron oxidation at potentials greater than ~1.25 V versus a Ag/Ag+ pseudo reference electrode.
Figure 1 shows two cycles of film growth. Cycle 1 (blue curve) shows only background (non-Faradaic) current until the potential is greater than 1.25 V. Cycle 2 shows additional Faradaic current beginning at approximately 0.75 V due to oxidation of the polymer film. Polymerization still happens at potentials greater than 1.25 V. The small spike at 1.1 V is related to an irreversible film rearrangement since subsequent cycles (seen when thicker films were prepared) show no current spike.
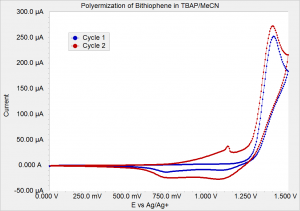
Figure 1: Electropolymerization of 1 mM bithiophene in 0.1 M TBAP/MeCN. Scan rate was 50 mV/s.
Once the film was deposited, the cell was washed with MeCN and then refilled with monomer-free electrolyte. Cycling of the polymer film revealed a broadly shaped couple at approximately 1 V as shown in Figure 2. Mass increases during oxidation and decreases during reduction, respectively, yet does not return to the initial value indicating some loss of solvent, electrolyte or polymer film.

Figure 2: Mass and Current versus Voltage for one cycle of polybithiophene in 0.1 M TBAP/MeCN. Scan rate was 100 mV/s
Echem Analyst™ calculates mass changes based on dFseries, the change in series resonant frequency, using the rewritten Sauerbrey1 equation below
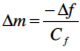
where Δf is the change in frequency and Cf is the theoretical calibration factor, 226 Hz cm2/µg, for a 10 MHz AT-cut quartz crystal. Mass change plotted against charge for a cycle in monomer-free electrolyte reveals information about mobile species during oxidation and reduction. Figure 3 shows the change in mass (Δm) versus the charge (C) for the first redox cycle.
Read complete article and download a PDF version at Gamry’s EQCM Investigations of a Thin Polymer Film application note.
