In the previous post (Electrochemical Corrosion Measurements Primer) we pointed out that Icorr cannot be measured directly. In many cases, you can estimate it from current versus voltage data. You can measure a log current versus potential curve over a range of about one half volt. The voltage scan is centered on Eoc. You then fit the measured data to a theoretical model of the corrosion process.
The model we will use for the corrosion process assumes that the rates of both the anodic and cathodic processes are controlled by the kinetics of the electron transfer reaction at the metal surface. This is generally the case for corrosion reactions. An electrochemical reaction under kinetic control obeys Equation 1-1, the Tafel Equation.
Equation 1-1
where,
I
= the current resulting from the reaction
I0
= a reaction dependent constant called the Exchange Current
E
= the electrode potential
Eo
= the equilibrium potential (constant for a given reaction)
β
= the reaction’s Tafel Constant (constant for a given reaction). Beta has units of volts/decade.
The Tafel equation describes the behavior of one isolated reaction. In a corrosion system, we have two opposing reactions – anodic and cathodic.
The Tafel equations for both the anodic and cathodic reactions in a corrosion system can be combined to generate the Butler-Volmer Equation (Equation 1-2).
![]()
where,
I
= the measured cell current in amps
Icorr
= the corrosion current in amps
E
= the electrode potential
Eoc
= the corrosion potential in volts
βa
= the anodic Beta Tafel Constant in volts/decade
βc
= the cathodic Beta Tafel Constant in volts/decade
What does Equation 1-2 predict about the current versus voltage curve? At Eoc, each exponential term equals one. The cell current is therefore zero, as you would expect. Near Eoc both exponential terms contribute to the overall current. Finally, as the potential is driven far from Eoc by the potentiostat, one exponential term predominates and the other term can be ignored. When this occurs, a plot of log current versus potential becomes a straight line.
A log I versus E plot is called a Tafel Plot. The Tafel Plot in Figure 1-1 was generated directly from the Butler-Volmer equation. Notice the linear sections of the cell current curve.
In practice, many corrosion systems are kinetically controlled and thus obey Equation 1.2. A log current versus potential curve that is linear on both sides of Ecorr is indicative of kinetic control for the system being studied. However, there can be complications, such as:
- Concentration polarization, where the rate of a reaction is controlled by the rate at which reactants arrive at the metal surface. Often cathodic reactions show concentration polarization at higher currents, when diffusion of oxygen or hydrogen ion is not fast enough to sustain the kinetically controlled rate. A more intuitive name for concentration polarization is “diffusion controlled”.
- Oxide formation, which may or may not lead to passivation, can alter the surface of the sample being tested. The original surface and the altered surface may have different values for the constants in Equation 1-2.
- Other effects that alter the surface, such as preferential dissolution of one alloy component, can also cause problems.
- A mixed control process where more than one cathodic, or anodic, reaction occurs simultaneously may complicate the model. An example of mixed control is the simultaneous reduction of oxygen and hydrogen ion.
- Finally, potential drop as a result of cell current flowing through the resistance of your cell solution causes errors in the kinetic model. This last effect, if it is not too severe, may be correctable via IR compensation in the potentiostat. For a good discussion on IR Compensation, see Gamry Instruments’ Application Note, Understanding IR Compensation .
In most cases, complications like those listed above will cause non-linearities in the Tafel Plot. The results derived from a Tafel Plot that does not have a well-defined linear region should be used with caution.
Classic Tafel analysis is performed by extrapolating the linear portions of a log current versus potential plot back to their intersection. See Figure 1-2 (this is Figure 1-1 reprinted with annotations that demonstrate the analysis). The value of either the anodic or the cathodic current at the intersection is Icorr. Unfortunately, many real world corrosion systems do not provide a sufficient linear region to permit accurate extrapolation. Most modern corrosion test software, such as Gamry Instruments’ DC105 DC Corrosion Techniques software, performs a more sophisticated numerical fit to the Butler-Volmer equation. The measured data is fit to Equation 1-2 by adjusting the values of Ecorr, Icorr, βa , and βc . The curve fitting method has the advantage that it does not require a fully developed linear portion of the curve.
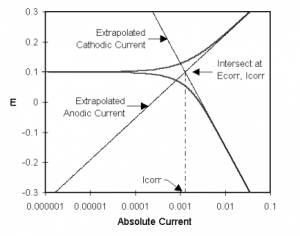
Figure 1-2. Classic Tafel Analysis.
To read the complete application note “Basics of Electrochemical Corrosion Measurements/Quantitative Corrosion Theory” please visit Gamry Instruments. You will also have an opportunity to download the complete note in PDF Format.
