Quantitative Corrosion Theory
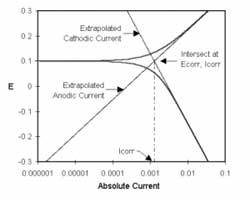
In the previous post (Electrochemical Corrosion Measurements Primer) we pointed out that Icorr cannot be measured directly. In many cases, you can estimate it from current versus voltage data. You can measure a log current versus potential curve over a range of about one half volt. The voltage scan is centered on Eoc. You then fit the measured data to a theoretical model of the corrosion process.
The model we will use for the corrosion process assumes that the rates of both the anodic and cathodic processes are controlled by the kinetics of the electron transfer reaction at the metal surface. This is generally the case for corrosion reactions. An electrochemical reaction under kinetic control obeys Equation 1-1, the Tafel Equation.
Equation 1-1