Using Digital Staircase Voltammetry
Introduction
Cyclic Voltammetry (CV) is unarguably the most popular electrochemical technique. It owes its well deserved reputation to its ability to deduce reaction mechanisms with relatively low cost equipment and quick experimentation. Since the very highly cited paper by Nicholson and Shain1 the technique has been the centerpiece of any electrochemical study.
CV involves sweeping the potential linearly between two limits at a given sweep rate while measuring current. The sweep rate chosen can be varied from few microvolts per second to millions of volts per second.
Electrochemical instrumentation has evolved vastly since the days of Nicholson and Shain. Currently, most manufacturers (including Gamry) make digital instruments with digital signal generators. These signal generators approximate the linear sweep with a staircase of variable step sizes and durations.
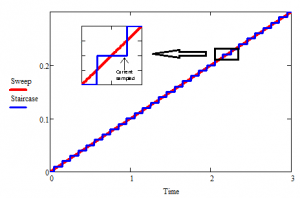
Figure 1 Staircase vs analog ramp
Early in the development of digital instruments (early 1990s), there have been reports analyzing the effects of using staircases instead of true ramps[2][3][4][5]. In staircase voltammetry, current can be sampled at various points during the step. Two of the report[2][3] investigated and tabulated the effects of this choice of sampling time. Another report[4] suggested the use of heavy analog filtering either on the applied or on the recorded signal as a way of eliminating the difference. The author demonstrated that through appropriately chosen analog filters, a ramp can be approximated by a staircase and the measured response is not adversely affected.
A later study[5] employed simulations to show that provided the potential steps are small enough, a staircase signal generated similar results as the linear ramp. The author investigated different coupled chemical reaction mechanisms including EC, CE, ECE, etc in addition to surface bound reactions like amalgamation and adsorption.
In this note, we will investigate the differences between data taken using a true analog ramp and a staircase using selected systems. We will show that, for experiments where faradaic reactions of solution species are studied, approximating a ramp with a staircase is adequate.
However, in experiments where faradaic reactions of surface species are involved, or when capacitance is measured, care has to be taken as there can be remarkable differences in the results. We will further introduce oversampling as an alternative solution and show that the oversampled and averaged data does not differ from that measured using the analog ramp.
Polycrystalline Platinum in Sulfuric Acid
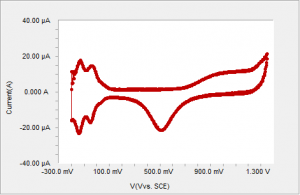
Figure 2 Typical voltammogram of polycrystalline Pt in sulfuric acid using an analog signal generator.
CV of polycrystalline platinum in dilute sulfuric acid produces strikingly different results depending on how the current is sampled. A typical voltammogram of polycrystalline platinum in sulfuric acid using a true analog ramp is shown on Figure 2.
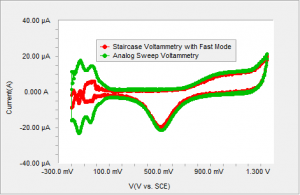
Figure 3 Polycrystalline Pt in sulfuric acid using staircase voltammetry with current sampled immediately prior to the step compared to the analog sweep.
There are several distinct regions in the CV profile. Starting from the negative end of the voltammogram, the first region of up to about 0.1 V is the adsorption (reductive peaks) and desorption of hydrogen. From 0.1 V to about 0.6 V no faradaic reactions occur. The third region is the oxide formation region starting at about 0.6 V6. The reduction of platinum oxide (negative currents) occurs at about 0.5 V. This voltammetry has been well studied and understood. The H-adsorption region, in particular, is often used as a tool to deduce the electrochemically active area of the Pt electrode.
Typically, in staircase voltammetry, a current reading is acquired immediately prior to the next step7. This method of sampling discriminates against any capacitive or surface bound reactions. The current due to any capacitive charging or faradaic current confined to the surface, decays in the initial part of the step and does not contribute to the measured current. Therefore, the hydrogen adsorption region is not well defined with the staircase voltammetry as shown in Figure 3.
Gamry employs a unique sampling mode to eliminate this difference. Instead of sampling the current at the end of the step where all the capacitive and surface related current has decayed, one can sample the current throughout the step and average. This way, the current due to capacitive and surface related effects are measured as well as any persistent effects throughout the step. This sampling mode is given the name “Surface Mode” in Gamry’s Framework Software. When surface mode sampling is used, structure in the data is recaptured as shown on Figure 4.
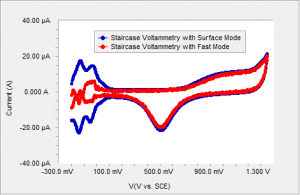
Figure 4 Polycrystalline Pt in sulfuric acid using surface mode sampling and fast mode sampling.
Double layer capacitance:
Read the complete application note. You will also have an opportunity to download this note in PDF format.
