EQCM Investigations
This Application Note is intended to provide the reader with a general framework for characterization of an electroactive polymer film. Electropolymerization is a convenient way to control film growth either through repeated cycling, potential steps, or current steps. Examination of the film redox behavior in monomer-free, fresh electrolyte provides insight on doping and dedoping of these polymer films.
Polybithiophene films were assembled by cycling an Au-coated 10 MHz quartz crystal between 0 and 1.5 V in the presence of 1 mM bithiophene solution containing 100 mM tetrabutylammonium perchlorate (TBAP) in acetonitrile (MeCN). Potentials are reported against a Ag/Ag+ pseudo-reference electrode. The Teflon® cell was outfitted with a Teflon o-ring to prevent swelling from the acetonitrile and placed inside a VistaShield™. The cell was connected to an eQCM 10M™ which was coupled to a Reference 600™. Both instruments were connected to a computer running Resonator™ version 5.67. Bithiophene, electropolymerizes via a two-electron oxidation at potentials greater than ~1.25 V versus a Ag/Ag+ pseudo reference electrode.
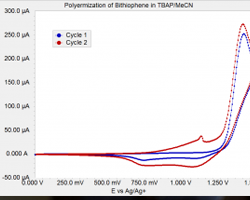
Figure 1 shows two cycles of film growth. Cycle 1 (blue curve) shows only background (non-Faradaic) current until the potential is greater than 1.25 V. Cycle 2 shows additional Faradaic current beginning at approximately 0.75 V due to oxidation of the polymer film. Polymerization still happens at potentials greater than 1.25 V. The small spike at 1.1 V is related to an irreversible film rearrangement since subsequent cycles (seen when thicker films were prepared) show no current spike.
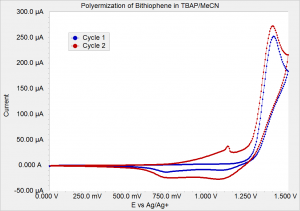
Figure 1: Electropolymerization of 1 mM bithiophene in 0.1 M TBAP/MeCN. Scan rate was 50 mV/s.
[…]