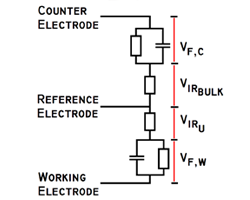
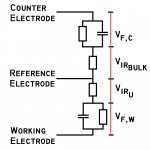
4 Terminal Measurements for EIS of Batteries-Coin Cell & Cylindrical
Purpose of This Note
This application note discusses the differences between 2‑point and 4‑point measurements of batteries. These two typical setups are compared with Gamry’s battery holders for CR2032 coin cells and cylindrical 18650 batteries. Both holders allow direct‑contact Kelvin sensing.
EIS measurements are performed with two types of lithium‑ion batteries and different experimental setups. In addition, shorted lead measurements show the low‑impedance limits of Gamry’s 18650 and CR2032 battery holders.
Introduction
It is crucial to know the exact specifications when testing batteries or any other energy storage device. Many parameters affect the capability of a battery, e.g. electrolyte, electrode materials, and temperature.
Batteries have to pass different tests to check their capacity, voltage window, current rating, internal impedance, leakage current, cycle life, operational temperature range, as well as several impact tests.
In order to get correct, reliable, and reproducible results, researchers have to rely on their experimental setup. Wrong setups can heavily affect and falsify measurement results leading to inaccurate conclusions.
The following sections show by means of EIS experiments the influence of the setup on the actual result. Common battery setups are compared with Gamry’s direct‑contact 4‑terminal battery holders. Shorted lead measurements with dummy cells show the lower limits of Gamry’s battery holders.
Dual Cell CR2032 and 18650 Battery Holder
Figure 1 shows typical options to connect cylindrical batteries and coin cells. Some batteries can be purchased with soldered tabs on each electrode. They allow connecting alligator clips for measurements. If no tabs are available, simple battery holders with two contacts are often used.
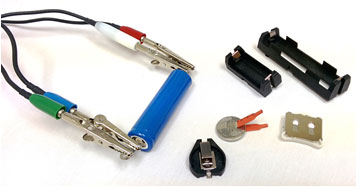
Figure 1 – Selection of battery connectors for cylindrical batteries and coin cells.