Basics of Electrochemical Impedance Spectroscopy
This application note presents an introduction to Electrochemical Impedance Spectroscopy (EIS) theory and has been kept as free from mathematics and electrical theory as possible. If you still find the material presented here difficult to understand, don’t stop reading. You will get useful information from this application note, even if you don’t follow all of the discussions.
Four major topics are covered in this Application Note.
- AC Circuit Theory and Representation of Complex Impedance Values
- Physical Electrochemistry and Circuit Elements
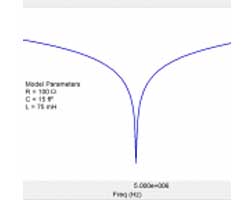
- Common Equivalent Circuit Models
- Extracting Model Parameters from Impedance Data
No prior knowledge of electrical circuit theory or electrochemistry is assumed. Each topic starts out at a quite elementary level, then proceeds to cover more advanced material.
AC Circuit Theory and Representation of Complex Impedance Values
Impedance Definition: Concept of Complex Impedance
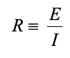
(1)
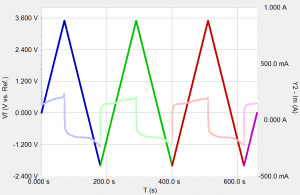
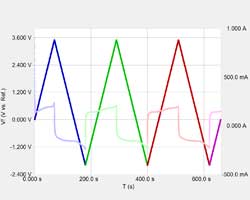
Almost everyone knows about the concept of electrical resistance. It is the ability of a circuit element to resist the flow of electrical current. Ohm’s law (Equation 1) defines resistance in terms of the ratio between voltage, E, and current, I.
While this is a well known relationship, its use is limited to only one circuit element — the ideal resistor. An ideal resistor has several simplifying properties:
- It follows Ohm’s Law at all current and voltage levels.
- Its resistance value is independent of frequency. AC current and voltage signals though a resistor are in phase with each other.


 what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.