Converting chemical energy into electrical energy can be done in different ways, but for chemical energy stored in various fuels like hydrogen, electrochemical fuel cells are the most direct, and have the potential to be very efficient.
The electrochemistry side of fuel cells is rather straightforward. Hydrogen, possibly with some carbon, on one side combines with oxygen on the other to produce water and possibly carbon dioxide. The half reactions are separated so that the energy difference between reagents and products can be harvested as electrical energy.
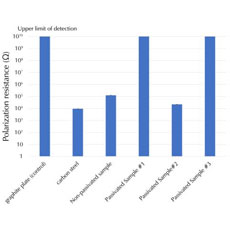
 Electrochemical Impedance Spectroscopy can identify problems that limit a fuel cell’s efficiency, by helping to optimize a cell, it can determine anodic and cathodic process mechanisms. EIS is particularly good for measuring the equivalent series resistance of fuel cells, a major source of power loss in a low impedance device. Because of the modeling capability of EIS, you can also extract information on kinetics and mass transport in the fuel cell, both of which are crucial factors to fuel cell performance. EIS is useful in both research and QC applications. EIS of fuel cells runs into some of the same low impedance device and setup limitations that also show up in batteries and supercapacitors.
Electrochemical Impedance Spectroscopy can identify problems that limit a fuel cell’s efficiency, by helping to optimize a cell, it can determine anodic and cathodic process mechanisms. EIS is particularly good for measuring the equivalent series resistance of fuel cells, a major source of power loss in a low impedance device. Because of the modeling capability of EIS, you can also extract information on kinetics and mass transport in the fuel cell, both of which are crucial factors to fuel cell performance. EIS is useful in both research and QC applications. EIS of fuel cells runs into some of the same low impedance device and setup limitations that also show up in batteries and supercapacitors.
[…]










 Electrochemical Impedance Spectroscopy can identify problems that limit a fuel cell’s efficiency, by helping to optimize a cell, it can determine anodic and cathodic process mechanisms. EIS is particularly good for measuring the equivalent series resistance of fuel cells, a major source of power loss in a low impedance device. Because of the modeling capability of EIS, you can also extract information on kinetics and mass transport in the fuel cell, both of which are crucial factors to fuel cell performance. EIS is useful in both research and QC applications. EIS of fuel cells runs into some of the same low impedance device and setup limitations that also show up in batteries and supercapacitors.
Electrochemical Impedance Spectroscopy can identify problems that limit a fuel cell’s efficiency, by helping to optimize a cell, it can determine anodic and cathodic process mechanisms. EIS is particularly good for measuring the equivalent series resistance of fuel cells, a major source of power loss in a low impedance device. Because of the modeling capability of EIS, you can also extract information on kinetics and mass transport in the fuel cell, both of which are crucial factors to fuel cell performance. EIS is useful in both research and QC applications. EIS of fuel cells runs into some of the same low impedance device and setup limitations that also show up in batteries and supercapacitors.