Being able to scan rapidly does not insure that the results will be meaningful! The speed of the current measurement circuitry is often the limiting factor!
The key to finding the practical limit for obtaining meaningful fast scan cyclic voltammograms is nearly always finding the speed of the current measurement. Here the researcher has an important role to play: It is the researcher who must select the current range to use for fast cyclic voltammetry. The autoranging capability of many modern computer controlled potentiostats generally cannot be used because the decisions cannot be made and implemented fast enough.
Because of stray (and deliberately added) capacitances, the current measuring circuitry generally becomes slower as the full scale current decreases. Obtaining the fastest scan requires a tradeoff of scan rate, electrode size, analyte concentration, current range, and acceptable noise in the measurement. It is often better to use a less sensitive current scale (larger full scale current) coupled with a larger pre-amplification factor on the ADC, data recorder, or oscilloscope used. Although this approach is likely to increase the noise in the measurement, it does allow a higher scan rate to be realized.
The speed or frequency response of each current range can sometimes be found in the manufacturer’s data sheet under “Current Measurement” or sometimes as a “System Specification” if a specific current range is quoted along with the bandwidth.
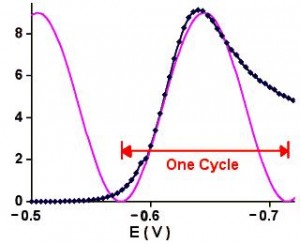
This number can be roughly translated into a scan rate by looking at Figure 1.
[…]

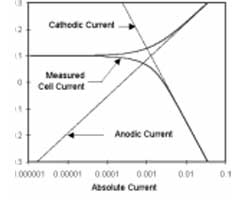
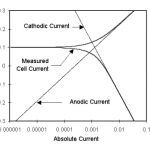

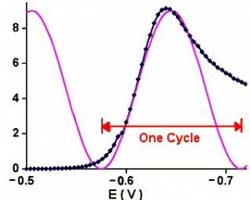


 One guideline that I have heard recommended (although I cannot give a reference for it) is that data over a decade range of frequency is required to support each circuit component.
One guideline that I have heard recommended (although I cannot give a reference for it) is that data over a decade range of frequency is required to support each circuit component.

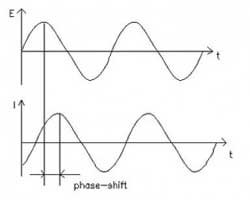
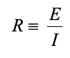

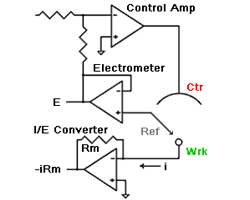
 what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.
what they study. However, the reference electrode shouldn’t be ignored. Its characteristics can greatly influence electrochemical measurements. In some cases, an apparently “good” reference electrode can cause a complete failure of the system.